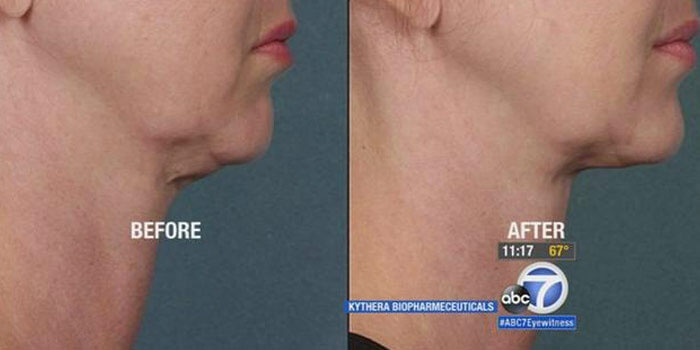
What is Kybella®
Kybella® is a new injectable medication unanimously supported by the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee and recently approved for treatment of moderate to severe submental fat in adults, also known as submental fullness or a double chin.
The new drug is the first non-surgical treatment approved by the agency for this indication. Before FDA approval of Kybella, patients troubled by a double chin could only opt for liposuction or surgery, invasive procedures that are significantly more expensive. The new injectable compound is a patented synthetic form of deoxycholic acid, a molecule that occurs naturally and aids in metabolism of dietary fat.
Kybella treatments now available at Mayoral Dermatology.
How does Kybella® work?
Submental fat is stored in fat cells that accumulate in the chin area. Kybella® has been described as a “fat-melting” compound, which is not completely accurate. It works by disrupting fat cell membranes, which causes cell destruction. This process is also known as cell “lysis.” When the membrane of the cell is destroyed, the fatty contents are released into the bloodstream and naturally metabolized by the body.
Kybella® is the first injection approved in the United States to dissolve fat cells.
Mesotherapy refers to a practice developed in France several decades before the advent of Kybella®. A variety of fat-dissolving mixtures are used in mesotherapy, containing deoxycholic acid and other ingredients, including herbal extracts and different acids. These mixtures have not undergone clinical trials and their effectiveness remains in question. The FDA approved Kybella® after proof of efficacy was demonstrated in well-designed clinical trials.
Who can administer Kybella® for treatment of double chin?
Dr. Flor Mayoral, Dr. Janelle Vega and Dr. Adriane Pompa have been selected and trained to inject Kybella for the treatment of double chin. The FDA approval stipulates administration by a licensed healthcare professional. Kythera Biopharmaceuticals, the company that developed this unique solution for submental fat, will begin training dermatologists and plastic surgeons in June. It’s important to choose a cosmetic physician with training in the use of this injection to avoid complications and achieve optimal results in the appearance of your double chin.
Who is a candidate for Kybella® treatment?
Kybella® is approved for adults over the age of 18 who wish to improve the appearance of submental fat deposits. It’s important to see a qualified physician for evaluation, since some patients may have submental fullness that is the result of an enlarged thyroid gland or enlarged lymph nodes and not due to fat. Patients with excessive skin under the chin may not be good candidates. Kybella® should not be administered to patients with an infection in the treatment area. The FDA suggests caution when considering Kybella® for use in patients who have had other surgeries or cosmetic treatments in the submental region. An experienced cosmetic dermatologist can help you determine if Kybella® is right for treatment of your double chin.
What to expect with Kybella®
Treatment with Kybella® may require up to 50 injections in the submental region in a single session. Up to six treatments may be required, and they should be administered at intervals of no less than one month apart. Topical numbing medicine and local anesthetics will make the procedure more comfortable. Because this treatment is not invasive, it can be done in the office and should take less than 30 minutes. The recovery time is short when compared to liposuction or surgery, although common post-treatment effects include swelling, bruising, pain, numbness, redness, and areas of hardness in the treated region. The treatment does not require maintenance, as destruction of fat cells is permanent. Doctors who participated in the clinical trial say changes will be visible within a few weeks of the first treatment.
Possible adverse effects
Kybella® is approved only for use in the submental area. Common side effects like swelling or bruising should resolve within a few days. There is a risk of serious side effects that include nerve injury in the jaw. This can result in facial weakness or asymmetry. It may result in difficulty with swallowing. For this reason, it’s essential to choose a highly qualified cosmetic physician to perform the procedure. Other healthcare professionals may not have adequate familiarity with variations in anatomy in the area of the chin and jaw.
Kybella® can destroy skin cells if it is injected into the dermis of the skin, which is another reason to choose an experienced cosmetic physician for administration of this treatment. Patients with severe double chin may develop excessive loose skin after treatment of the submental fat. This can be improved with surgical tightening procedures or by use of non-invasive treatments.
Can Kybella® be used to reduce other areas of body fat?
Kybella® has only been approved for treatment of submental fat deposits. The safety and efficacy of Kybella® in other regions of the body are not known, and the manufacturer cautions against unapproved uses.
How expensive is treatment with Kybella®?
Kythera Biopharmaceuticals has not yet released a shelf price for the new drug, but it will be much less expensive than liposuction or surgery for treatment of a double chin. The company expects to release more information about pricing after the official launch.
When choosing this treatment, remember that compounded injections containing deoxycholic acid are not approved by the FDA, and you should be certain your doctor is using the product manufactured by Kythera. Other preparations have been associated with serious side effects in the past.